
Insights+: The US FDA New Drug Approvals in December 2023
Shots:
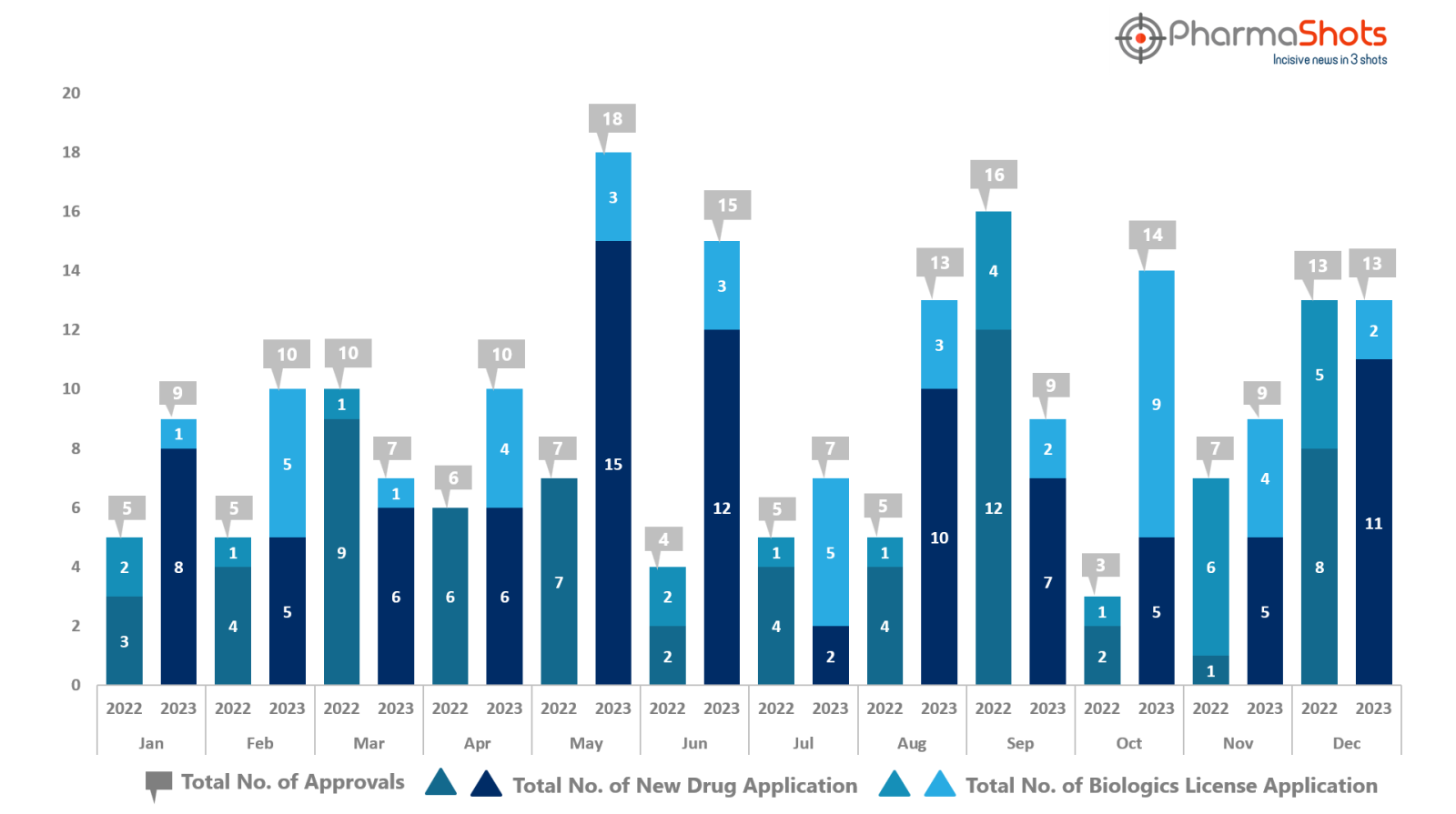
- The US FDA approved 11 NDAs & 2 BLAs in December 2023, leading to treatments for patients and advances in the healthcare industry. The CDER and CBER approved 109 novel products in 2023
- In December 2023, the major highlight drugs were Jaypirca (pirtobrutinib) approved for Chronic Lymphocytic Leukemia or Small Lymphocytic Lymphoma, and Iwilfin (belzutifan) for the Treatment of Renal Cell Carcinoma
- PharmaShots has compiled a list of US FDA approved drugs in the month December 2023
Active ingredient: Pirtobrutinib
Approved: Dec 4, 2023
Company: Eli Lilly
Disease: Chronic Lymphocytic Leukemia or Small Lymphocytic Lymphoma
- The approval was based on the P-I/II (BRUIN) study evaluating the efficacy of Jayprica (200mg; QD) for the treatment of patients (n=108) with CLL/SLL who were previously treated with at least two prior lines of therapy excl. CNS involvement or recent HSCT
- The result showed ORR & partial response of 72% whereas the time to response was 3.7 mos. & mDoR was 12.2 mos. Additionally, ARs led to dose reductions in 3.6%, treatment interruption in 42%, & permanent discontinuation in 9%
- Jaypirca (pirtobrutinib) approved under the FDA's Accelerated Approval is a reversible BTK inhibitor, targeting B-cell cancers like mantle cell lymphoma and CLL/SLL
Active ingredient: Iptacopan
Approved: Dec 6, 2023
Company: Novartis
Disease: Paroxysmal Nocturnal Hemoglobinuria
- The approval was based on the P-III (APPLY-PNH) & (APPOINT-PNH) trials evaluating Fabhalta (200mg, oral, BID) vs anti-C5 therapies & Fabhalta alone in PNH patients with residual anemia & those naïve to complement inhibitor (incl. anti-C5 therapies) respectively for 24wks.
- The results depicted that of the patients with a sustained increase of Hb levels ≥2g/dL from baseline in the absence of transfusions, responses were seen in 82.3% vs 0% of patients in (APPLY-PNH) & 77.5% of patients in (APPOINT-PNH) study
- Furthermore, of the patients with a sustained increase of Hb levels ≥12g/dL in the absence of transfusions, responses were seen in 67.7% vs 0% of patients with a transfusion avoidance rate of 95.2% vs 45.7% in the (APPLY-PNH) study
Active ingredient: Isavuconazole
Approved: Dec 11, 2023
Company: Basilea Pharmaceutica
Disease: Invasive Aspergillosis and Invasive Mucormycosis
- The US FDA approves the expanded use of Antifungal Cresemba in the US in Children with Invasive aspergillosis (IA) & invasive mucormycosis (IM)
- The approval was granted based on the outcomes of two pediatric clinical studies, incl. a P-II study evaluating the safety, effectiveness, & PK of Cresemba in pediatric patients aged 1 to 17 years, for the treatment of IA and IM
- Additionally, the US FDA granted Cresemba pediatric exclusivity, extending U.S. market exclusivity to Sept 2027. In Europe, a similar application awaits EMA assessment, with a decision expected in H1’24. Cresemba is approved in 76 countries and marketed in 71, incl. the US & most EU states
4. US WorlMeds' Iwilfin Receives the US FDA's Approval for the Treatment of Children with Neuroblastoma
Active Ingredient: Iwilfin
Approved: Dec 14, 2023
Company: US WorlMeds
Disease: Neuroblastoma
- The approval was based on the clinical evaluation of Iwilfin (192mg, oral, BID) as maintenance therapy following SoC treatment, including immunotx. vs external control in children with high-risk neuroblastoma
- The results from the study depicted an improved EFS & OS whereas in 4yrs. following immunotx., the EFS was 84% vs 73%, 96% vs 84% of patients were alive thereby depicting a reduction in risk of relapse & death by 52% & 68%
- On further analysis the confirmatory results depicted a reduction in risk of relapse & death by 57%-41% & 71%-55%. Additionally, US WorldMeds partnered with the Beat Childhood Cancer Research Consortium to conduct the preclinical & clinical evaluation of Iwilfin
Active Ingredient: Belzutifan
Approved: Dec 15, 2023
Company: Merck & Co.
Disease: Renal Cell Carcinoma
- Merck received the US FDA’s approval based on the results from the P-III (LITESPARK-005) clinical trial evaluating the safety & efficacy of Welireg (120mg, QD) vs everolimus (10mg, QD) in patients (n=746), in the ratio 1:1, with advanced RCC that progressed following PD-1 or PD-L1 checkpoint inhibitor & VEGF receptor-targeted therapies
- As per the results, the trial depicted a PFS of 5.6mos. vs 5.6mos., ORR of 22% vs 4%, CR of 3% vs not achieved with everolimus & PR of 19% vs 4%
- Moreover, out of the 82 patients achieving confirmed responses on receiving Welireg (HIF-2α inhibitor), 30% showed a DoR of ≥12mos. whereas disease progression was reduced by 25% & mDoE was 7.6mos. in patients receiving Welireg
Active Ingredient: Travoprost
Approved: Dec 15, 2023
Company: Glaukos
Disease: Open-Angle Glaucoma (OAG)
- The approval was based on the results from 2 P-III trials incl. (GC-010) & (GC-012) evaluating the safety & efficacy of iDose TR (75 mcg) models with varying travoprost release rates vs topical timolol ophthalmic solution (0.5%, BID) in lowering IOP in patients (n=1,150) suffering from OHT or OAG
- In both trials, 98% vs 95% of patients continued evaluation, and 81% of iDose TR patients were fully free of topical medicines that lower IOP at 12mos. The approval was also supported by the P-IIb study results that depicted a reduction in topical IOP-lowering for up to 36mos.
- Glaukos expects to initiate the commercial launch activities for iDose TR by Q1’24 & has established a wholesale acquisition cost of $13,950/dose or implant
7. Merck’s Keytruda + Padcev Received the US FDA Approval for the Treatment of Urothelial Cancer
Active Ingredients: Pembrolizumab & Enfortumab
Approved: Dec 15, 2023
Company: Merck & Co.
Disease: Urothelial Cancer
- The approval was based on the P-III (KEYNOTE-A39) trial evaluating Keytruda + Padcev vs CT (gemcitabine + cisplatin/carboplatin) in patients (n=886) with locally advanced or metastatic urothelial cancer. The result of the study demonstrated statistically significant improvement in OS & PFS
- Additionally, the result also demonstrated a median OS of 31.5 vs 16.1mos. along with a reduced risk of death by 53% & disease progression by 55%. Median PFS was 12.5 vs 6.3mos. & ORR was 68% vs 44%. Furthermore, CR was found to be 29% vs 12%, & PR was 39% vs 32%
- Keytruda, humanized mAb activates T lymphocytes, which may have an impact on both tumor cells and healthy cells, by interfering with the interaction between PD-1 and its ligands, PD-L1 and PD-L2
Active Ingredient: Roflumilast
Approved: Dec 18, 2023
Company: Arcutis Biotherapeutics
Disease: Seborrheic Dermatitis
- The approval was supported by P-II (Trial 203) & P-III (STRATUM) study evaluating the safety & efficacy of Zoryve foam, 0.3% vs Vehicle in patients (n=683) with seborrheic dermatitis
- The P-III (STRATUM) study met its 1EP that showed IGA success rate in nearly 80% of individuals vs 58% by 8 wks. Additionally, significant improvements were observed in all 2EPs, incl. itch, scaling, & erythema. More than 60% of individuals experienced a ≥4-point reduction in itch by Week 8
- In addition, more than 50% of individuals achieved an erythema score of 0, & more than 50% achieved a scaling score of 0 at 8wks. In P-II (Trial 203), Zoryve foam exhibited IGA Success rate in 73% of individuals vs 40.8%
Active ingredient: Immune Globulin
Approved: Dec 18, 2023
Company: GC Biopharma
Disease: Primary Humoral Immunodeficiency
- The Approval was based on the P-III study evaluating the safety & efficacy of GC5107B for the treatment of adult patients aged 17 years & older with primary humoral immunodeficiency (PI)
- The result of the study depicted 1EP of 0.03 aSBIs per patient-year, meeting the FDA requirement of <1 aSBI per patient-year. The proportion of infusions with AEs within 72 hrs. after the infusion was 0.22, meeting the FDA-required endpoint of less than 0.40
- Alyglo, a 10% IgG liquid for IV infusion, is sourced from human plasma. Its manufacturing process incl. fractionation, solvent/detergent treatment, and nanofiltration.Cation Exchange Chromatography (CEX) is employed in the process
10. Chiesi Receives the US FDA's Approval for Filsuvez as a Treatment for Epidermolysis Bullosa (EB)
Active ingredient: Filsuvez
Approved: Dec 19, 2023
Company: Chiesi
Disease: Epidermolysis Bullosa
- Filsuvez was evaluated vs control gel in a P-III (EASE) clinical trial in patients (n=223) with EB (wound size between 10-50cm2) across 58 sites in 28 countries. The 1EP of the study incl. efficacy of Filsuvez vs control gel
- The study met both its 1EP & 2EP by depicting statistically significant results along with favorable differences between Filsuvez and control gel. Moreover, Chiesi received the rights to Filsuvez through its acquisition of Amryt Pharma in Jan 2023
- Filsuvez is a topical gel used for the treatment of adults and children aged 6mos.or older with EB & has been approved by the EMA in Jun 2022 for both JEB & DEB
Active ingredient: Budesonide
Approved: Dec 21, 2023
Company: Calliditas Therapeutics
Disease: IgA Nephropathy
- Followed by the accelerated approval in Dec 2021, the US FDA has now granted full approval to the company’s Tarpeyo for the treatment of IgA nephropathy (IgAN) based on a measure of kidney function
- The approval was supported by the results from the P-III (NefIgArd) study to investigate the efficacy and safety of Tarpeyo (16mg, QD) vs PBO for the treatment of patients (n=364) with primary IgAN as an optimized RASi therapy
- The impact of Tarpeyo on eGFR was evident by 3mos. and remained consistent over 2yrs., with a 5.9 mL/min/1.73 m2 difference in mean change from baseline vs PBO at the end of year 2. The observed effect on kidney function during the 9mos. treatment period persisted beyond treatment completion throughout the study
Active Ingredient: Eplontersen
Approved: Dec 22, 2023
Company: AstraZeneca
Disease: Polyneuropathy of Hereditary Transthyretin-Mediated Amyloidosis
- The approval was based on the P-III (NEURO-TTRansform) clinical trial evaluating the safety & efficacy of Wainua vs PBO in patients with ATTRv-PN (Stage 1 or 2)
- The results portrayed that patients treated with Wainua depicted consistent & sustained benefits on co-primary endpoints (serum TTR concentration & neuropathy impairment measures by mNIS+7) & key secondary endpoints of QoL on Norfolk QoL-DN. These results were published in the JAMA
- Following AstraZeneca & Ionis’ global development & commercialization agreement, both companies will commercialize Wainua across the US & will seek regulatory approval in the EU& other parts of the world. The company expects to make Wainua available in the US market by Jan 2024
13. The US FDA Approves BeiGene’s Label Update for Brukinsa to Include Chronic Lymphocytic Leukemia
Active Ingredient: Zanubrutinib
Approved: Dec 25, 2023
Company: BieGene
Disease: Chronic Lymphocytic Leukemia
- The label expansion was based on the results from the P-III (ALPINE) clinical trial evaluating the safety & efficacy of Brukinsa vs Imbruvica in patients previously treated with r/r chronic lymphocytic leukemia (CLL)
- With a median follow-up of 31mos. the study depicted a superior PFS & demonstrated a favorable cardiac safety profile with significantly lower rates of atrial fibrillation/flutter (5.2% vs 13.3%) & lower deaths (0 vs 6) in patients receiving Brukinsa vs Imbruvica
- These results were presented at the ASH 2023 & were presented in The New England Journal of Medicine. Brukinsa is a small molecule inhibitor of BTK designed to deliver complete and sustained inhibition of the BTK protein
Related Post: Insights+: The US FDA New Drug Approvals in November 2023
Tags

Shivani was a content writer at PharmaShots. She has a keen interest in recent innovations in the life sciences industry. She was covering news related to Product approvals, clinical trial results, and updates. We can be contacted at connect@pharmashots.com.














